The need for cleaner energy systems, including CO2 capture technologies, is driving the current development of chemical looping technologies such as chemical looping combustion (CLC) and chemical looping gasification (CLG). Chemical looping is based on using oxygen carriers (e.g., iron oxides, copper oxides, calcium sulfates, etc.) to provide the oxygen for the reaction with the fuel.
To optimize the overall process performance, it is critical that the properties of the oxygen carriers are well-defined and maintained for their specific purpose during the different stages of the CLC process. One of the critical properties of the oxygen carrier is its oxidation state (e.g., content of Fe2O3 vs. Fe3O4) as it affects the fundamental operation of the CLC process. Unfortunately, the ability to make on-line measurements of the oxidation state of oxygen carriers is lacking and new sensors need to be developed.
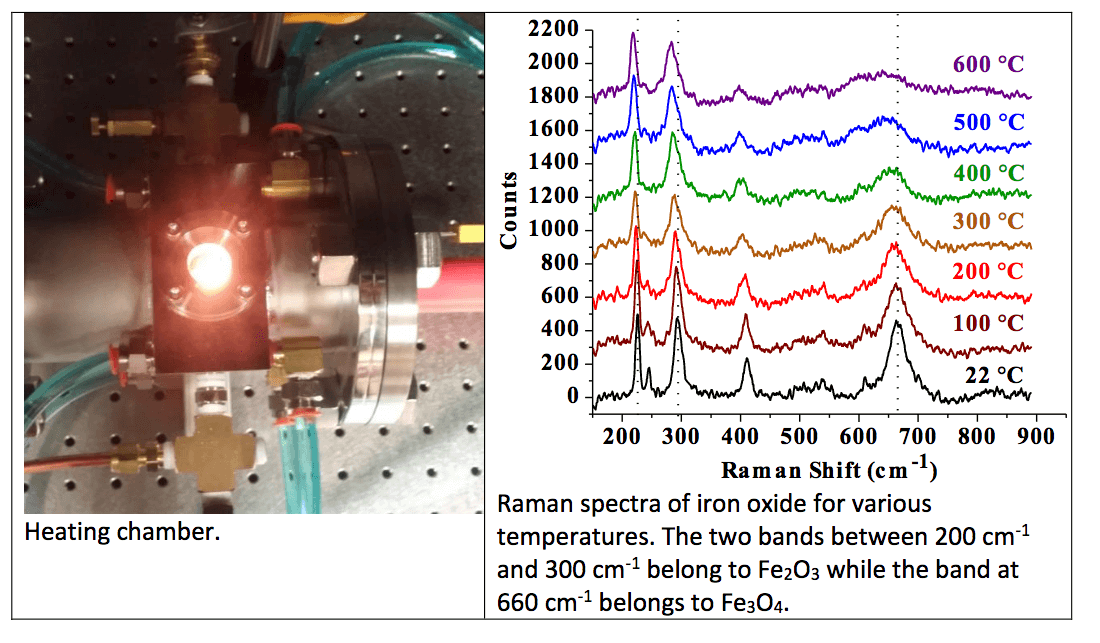
Using a high-temperature sample chamber, ASL evaluated the potential of Raman spectroscopy as a sensor for the on-line analysis of the oxidation state of oxygen carrier particles. The results indicate that this technique is capable of distinguishing mixtures of Fe2O3 and Fe3O4 for temperatures up to 600 °C.